List of Contents
What is Point of Care Testing Market Size?
The global point of care testing market size is estimated at USD 44.48 billion in 2025 and is anticipated to reach around USD 125.33 billion by 2034, expanding at a CAGR of 12.20% from 2025 to 2034.
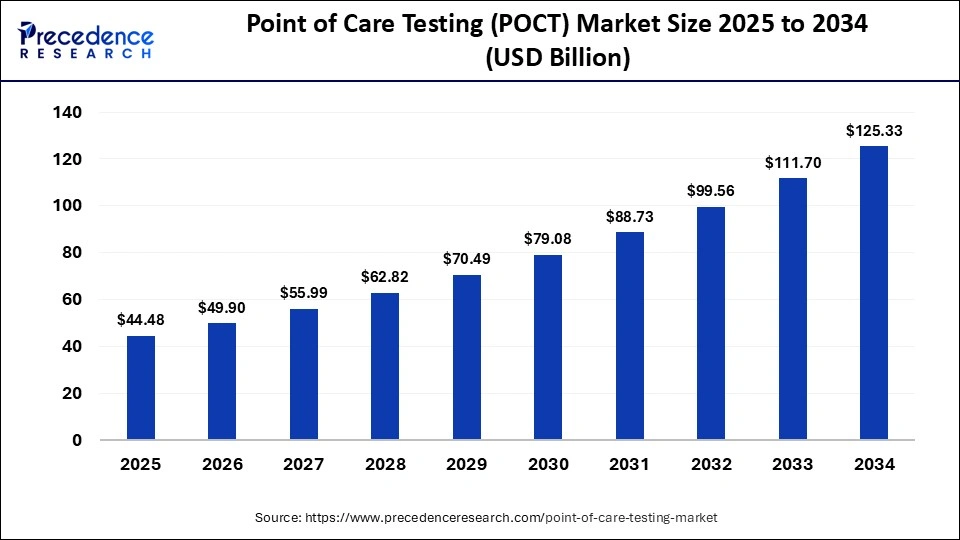
Market Highlights
- North America was accounted highest revenue share more than 46% in 2024.
- Asia Pacific POCT market is registering at a CAGR of 12.38% from 2025 to 2034.
- By product, glucose monitoring products segment has captured highest revenue share 27% in 2024.
- By platform, lateral flow assays segment has accounted 36% revenue share in 2024.
- By mode of purchase, OTC testing products segment has held 59% revenue share in 2024.
- The glucose monitoring segment was valued at USD 8.91 billion in in 2024.
- The biosensors segment is growing a CAGR of 9.2% during the frorecast period.
- The pregnancy and fertility testing products segment is expected to reach at a 8.1% of CAGR from 2025 to 2034.
- The diagnostic centers segment is growing at a CAGR of 7.8% over the forecast period.
What is point-of-care testing?
Point-of-care testing markets are basically utilised for diagnosis of the disorder by performing various tests at the proper place and the proper time with increased facilities for testing the disorder with the availability of the testing kits available in the market. Point-of-care test markets are very normal tests and easy that can patient itself also can perform the test sitting at one place. Developing technologies in the medical sector with the aim of patients health and increased demands from the medical sector with rising disorders among the population and for fast treatment and quick diagnosis of the disorder available for quick treatment to the patients.
Diagnostic kits such as urine test strips available in the market for detecting the disorder or any problem. Pulse oximetery the electronic device that patient can check the oxygen level sitting at home and many more test are performed and in the laboratory with increased demands for glucose check, haemoglobin check and more mover. Which helped to expand the market of point care testing market.
Impact of COVID-19 on the point of care testing market rate with increased market revenue share due increasing number of patients with the viral infection and wide spread of corona virus affecting the patients health with increased demands from the medical sector for rapid testing and antigen testing of the patient and increased demands for blood testing, x-ray and many more test which are required for detecting the spread of disease with improved number of diagnostic procedure available which help to enhance the market of point care testing market.
Point of Care Testing Market Growth Factors
Point-of-care testing are the medical devices utilized for diagnosis of the disorder and faster results with increased efficiency and accuracy of the diagnostic kits with increased demands from the market so that people sitting at home any time any place can access the kit with quick results and fast treatment with the increased focus with increasing care of the patient and enhance the market growth of market due to increased demands.
Increasing prevalence of chronic disorders among the patients with disorders such as cancer, diabetes, arthritis, cardiovascular disorder and other with increased medical diagnostic kits available in the market and increased number of patients for testing purpose with increased technology and developed techniques in point-of-care testing market with increased research and development with new innovative techniques involved with increased software system and increased connectivity for performing the tests led to enhance the market rate of point care testing market.
The use of different point-of-care testing procedures and devices available in the market with different disorder accelerate the market. The key market players involved in the introduction of new launches in medical diagnostic kits with increased research and development and increased investment from the key market players help to grow the market of market. Increased interference of the government with increased funding for research and development of new techniques and enhance number of policies of reimbursement helps to drive the market.
The outbreak of COVID-19 with increased spread of corona virus with positive and negative impact on the point care testing market with increased demands from the health sector with rising patients which propel the point-of-care testing market at increased CAGR rate.
Point-of-Care Testing (POCT) Market Outlook
- Industry Growth Overview: The POC testing market is poised for significant growth from 2025 to 2030, driven by a combination of increasing demand for rapid diagnostics and advances in technology, acceleration of POC testing in infectious diseases, chronic disease conditions, and urgent care. This growth, in the aggregate, will be strongest in North America and the Asia-Pacific regions due to the expansion of healthcare systems and rising awareness of patients regarding testing of their ailments.
- Sustainability Trends: The POC testing market is shifting towards eco-friendly and disposable POC testing kits, with some manufacturers looking to reduce plastic use while others are moving to materials that are recyclable. Companies such as Abbott and Roche are investing in reducing plastic utilization in production, using biodegradable components and materials that comply with environmental regulations.
- Global Expansion: Leading companies in their sector are expanding their operations into emerging markets, specifically Southeast Asia, Latin America, and the Middle East. Companies like Siemens Healthineers and Quidel are developing regional manufacturing and distribution systems to better enable access and decrease turnaround times.
- Key Investors:Both strategic and private equity investors have grown their investments, encouraged by attractive margins, recurring consumables, and technology development. Recently, Temasek and Bain Capital have made investments in molecular and rapid diagnostics startups.
- Startup Ecosystem: The POC testing startup ecosystem is flourishing, especially in the areas of wearable diagnostics, AI-enabled devices, and multiplex testing. Several companies, such as Cue Health (USA) and Egenesis (Singapore), have attracted large rounds of VC funding to further innovative, patient-centric solutions.
Market Scope
| Report Coverage | Details |
| Market Size in 2025 |
USD 44.48 Billion |
| Market Size in 2026 |
USD 49.90 Billion |
| Market Size by 2034 |
USD 125.33 Billion |
| Growth Rate from 2025 to 2034 | CAGR of 12.20% |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Product Type, Platform, Mode of Purchase, Testing Type, End-User, and Geography |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
- Enhance prevalence of chronic disorders - Increasing number of disorders among the population with changing modern life style, dietary intake, obesity, lack of exercise led to increasing number of disorders and increased utilization of diagnostics kits and increased efficiency of the diagnostic kits with increased accuracy of the test drives the market of point care testing market. Chronic disorders such as diabetes, oncology, cardiovascular, arthritis, respiratory disorder and many more. Increased research and development with new technologies integrated in the medical sector and supply of needs required for curing the patients health with quick relief and quick results helps to drive the market of point care testing market.
- Developing infrastructure and health care facilities - Huge number of people with chronic disorders and increased demands from the market for medical diagnostic kits with increased technology and techniques involved for carrying out the procedures in the laboratory or at home with ease of access to the medical devices for detecting of the problem. Improved number of health facilities in the hospital with newly developed technologies and improved research and technology with new launches by the key market players helps to accelerate the market of market.
Key Market Challenges
- The rising cost of the point care testing medical devices - The rapid advance technologies developed in the market with increased production and manufacturing of the medical devices utilized in the hospitals for detection of the various disorders with increased efficiency and accuracy of the testing kit which led to increased cost of the testing procedures with newly developed testing kits. Enhance cost of the medical diagnostic devices can challenge the market to grow at higher value
- Lack of skill - The increasing medical diagnostic technologies in the market with improved techniques with software system embedded in to the devices which are installed in the hospital with increased connectivity. The new techniques require new skills lack of skill among the individuals enable to perform the medical diagnostic procedure. which may hamper the market rate of market
Key Market Opportunities
- Emerging underdeveloped regions with new developments and rising disorder - The rapid acceptance of the newly developed technologies in medical diagnostics for and enhance testing with improved technologies in the point care testing with increased efficiency and accuracy of the test. Development of point care testing across various regions with increased necessity of the testing kits drives the market of market to a larger extend with increased market share during the forecast period.
- Government interference with increased facilities - Increased government interference in the Point-of-care testing market with increased installation of the medical devices required for diagnosis of the disorder and increased providence from the government of various regions for introducing new techniques and technologies in Point-of-care testing market. Increased government policies such as reimbursement so that every individual can opt for the new devices developed and can able to the benefit. Which helps to accelerate the growth of market at considerable height.
- The key market players - Wide research and development programs carried out by the key market players with increasing investment for introducing new medical diagnostic kits with improved efficiency and efficacy with improved results and good response from the customer has emerged to be the major opportunity which helps to boost the market of point-of-care testing market during the forecast period.
Segment Insights
Product Type Insights
On the basis of product type, glucose monitoring product to hold the largest market share globally with increased demands from the customer with increasing disorders among the population and enhance demands for glucose monitoring product. Utilisation of glucose monitoring product use to determine the glucose level in individual which provides the information of insulin release in the body.
Glucose monitoring device is self-accessible to prick with needle on finger and collection of samples on the device and provides the information of glucose levels in the patients body. Utilisation of the point care testing with increased demands for the glucose monitoring device from the customer with diabetes-I and diabetes-II.
Platform Insights
On the basis of platform, lateral flow assay with highest market value in point care testing market. Increased analysis of the sample by immunochromatographic techniques for detection of analytes in the sample with detecting and quantifying of complex samples and submitting the results less than 30min period of time. Increased acceptance of the developed platforms and lateral flow assay test with conventual procedures performed in the labs helps to expand the market of Point-of-care testing market.
End-Users Insights
On the basis of end users, clinical laboratories accounted for the largest market share with increasing number of chronic disorder and increased utilization of testing sample in the laboratories. Covid-19 with wide spread of virus and increase in development of the testing and made available in the laboratories so that the individual can examine the presence of virus with enhance requirements from the market for point-of-care testing market. The home care testing services which are self-tested with highest market with increasing CAGR helps to drive the market.
Regional Insights
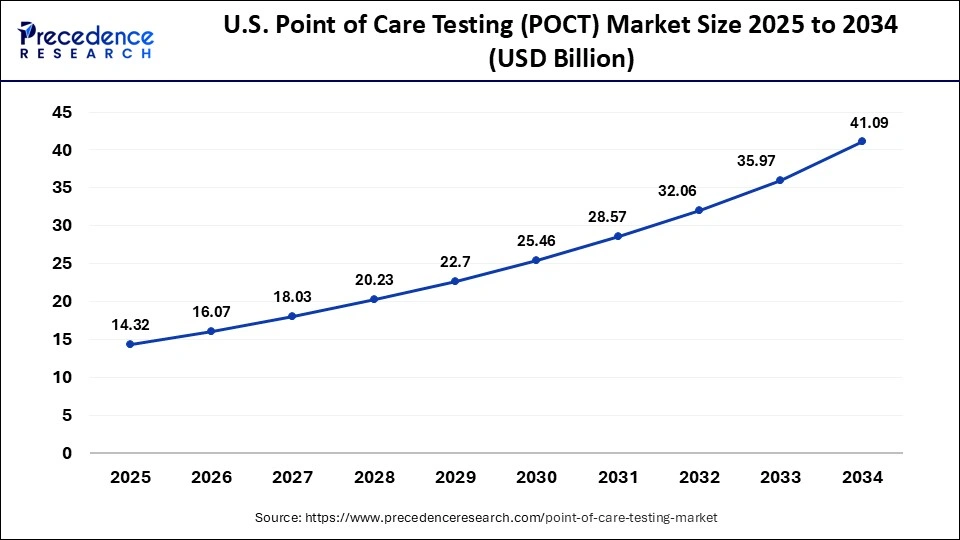
U.S. Point of Care Testing Market Size and Growth 2025 to 2034
The U.S. point of care testing market size is evaluated at USD 14.32 billion in 2025 and is predicted to be worth around USD 41.09 billion by 2034, rising at a CAGR of 12.38% from 2025 to 2034.
On the basis of geography, North America with highest market with increased CAGR in point-of-care testing market with increasing disorder among the population and increased demands for medical diagnostic kits with quick detection of the disorder and fast treatment increase the market revenue share of point-of-care test with developed technology with continuous research helps to drive the market.
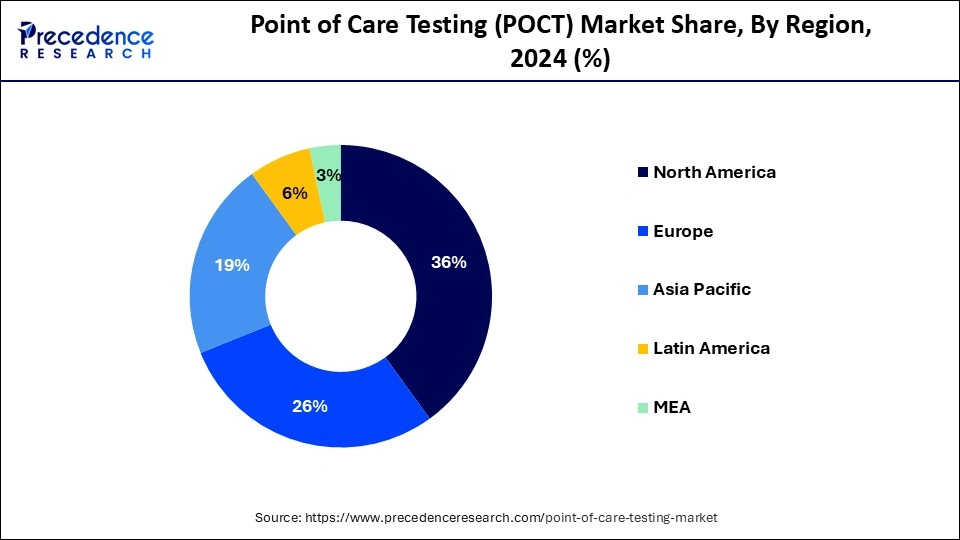
Other regions such as North America, Middle East and Africa, Asia Pacific, Europe in process to contribute the market share with increasing demands for point-of-care testing with increasing disorder with enhance market size.
Key Players in Point of Care Testing Market and their Offerings
- Abott Laboratories
- bioMrieux SA
- Becton Dickinson and Company
- Danaher Coraporation
- Nova Biomedical
- Sysmex corporation
- Johnson and Johnson
- Biosensor Inc
- Fluxergy
- Precision
- Ascensia Diabetes Care Holding AG
- LifeScan IP Holdings, LLC
- Mankind pharma
- OraSure Technologies, inc
- A. Menarini Diagnostics s.r.l
- ACON Laboratories
- Trinity Biotech plc
|
Company |
About |
|
Sinocare Inc |
Sinocare Inc was established in 2002. The firm develops diagnostic devices and smart health solutions that can support eco-friendly transportation by tracking driver health and minimizing fatigue or illness-related accidents. |
|
Hoffmann La Roche Ltd |
Hoffmann La Roche Ltd was incorporated in 1896. The company offers innovative healthcare diagnostics that facilitate safe transportation to support eco-friendly transit systems by facilitating healthier, more alert drivers and staff. |
|
Siemens AG |
Siemens AG was incorporated in 1847. The company creates smart mobility solutions, which include electric and automated transport systems, which assist cities with adopting eco-friendly transportation solutions to reduce pollution while at the same time increasing efficiency. |
Recent Developments
- In the year 2018 December, Anteo Diagnostics of Australia collaborated with Shanghai GeneoDx Biotechnology of china introduced the AnteoBind products with an agreement of sell and distribution of product in china.
- In the year 2017 January, Chembio Diagnostics Inc, (US) adopt the RVR diagnostics and expansion of the products with increased offers and availability of product in Southeast Asia.
- In the year 2016 March, Collaboration of Chembio Diagnostics Inc and BioManguinhos/Fiocruz (Brazil) for the development of Point-of-care diagnostic kit with the new launch of Zika test.
Segments Covered in the Report
By Product Type
- Covid -19 testing kits
- Glucose monitoring products
- Meters
- Strips
- Lancets and Lancing devices
- Cardiometabolic Monitoring Products
- Cardiac maker testing products
- Blood Gas / Electrolyte testing products
- HbA1c testing products
- Infectious disease testing products
- Influenza testing products
- HIV testing products
- Hepatitis testing products
- Sexually Transmitted Diseases testing products
- Healthcare - associated Infection testing products
- Respiratory infection testing products
- Tropical disease testing products
- others
- Coagulation monitoring products
- PT / INR testing
- ACT / APTT
- Pregnancy and fertility testing products
- Pregnancy testing products
- Fertility testing products
- Cancer marker testing products
- Urinanalysis testing products
- Cholestrol testing products
- Hematology testing products
- Drugs-of-abuse testing products
- Fecal Occult testing products
- Others
By Platform
- Lateral Flow Assays
- Dipsticks
- Microfluids
- Molecular Diagnostics
- Immunoassays
By Mode of Purchase
- OTC products
- Prescribed based products
By Testing Type
- Immunoassays
- Cell-Based Assays
- Nucleic Acid Amplification Testing
- Clinical Chemistry Assays
- Hematology
By End-User
- Clinical laboratories
- Ambulatory care facilities and physician offices
- Pharmacies, retail clinics & E-comm platforms
- Hospitals, Critical care centres, Urgent care centres
- Home care & self-testing
- Others
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client



