List of Contents
What is Pharmacovigilance Market Size?
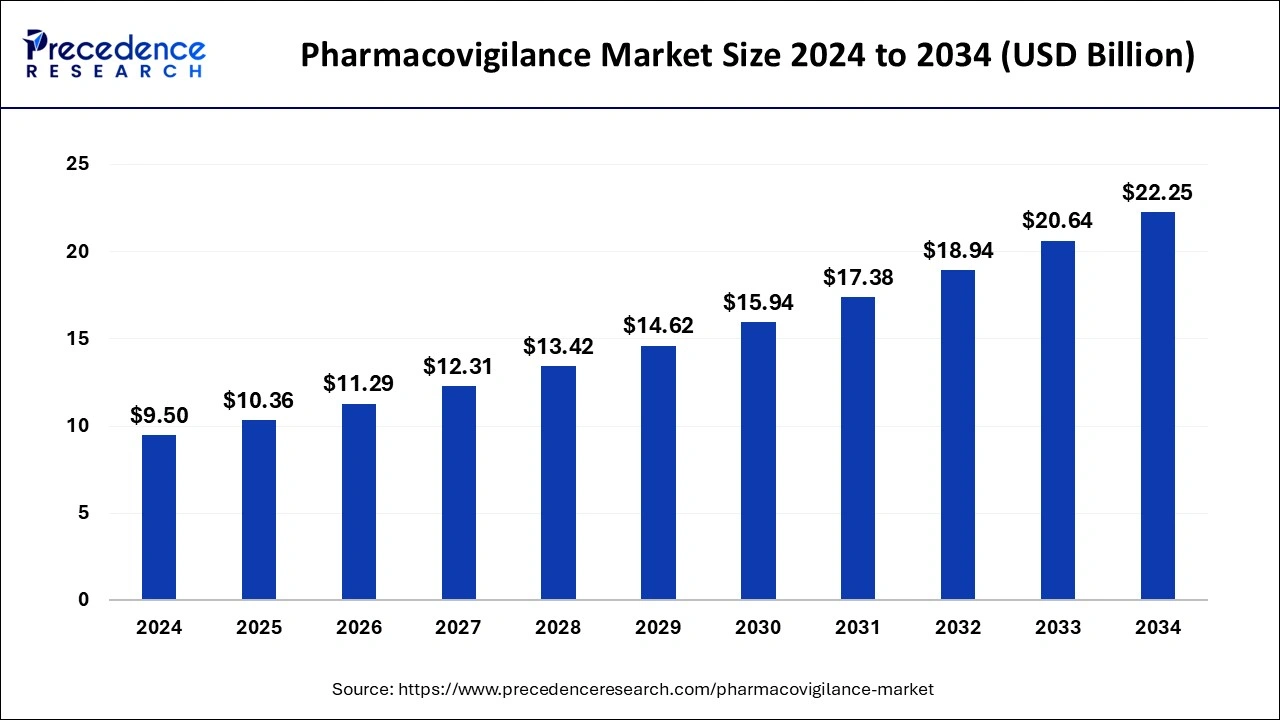
The global pharmacovigilance market size is estimated at USD 10.36 billion in 2025 and is expected to reach over USD 22.25 billion by 2034, poised to grow at a registered CAGR of 8.88% from 2025 to 2034. The growing incidences and prevalence of chronic diseases will continue aiding pharmacovigilance market in the forecast period.
Market Highlights
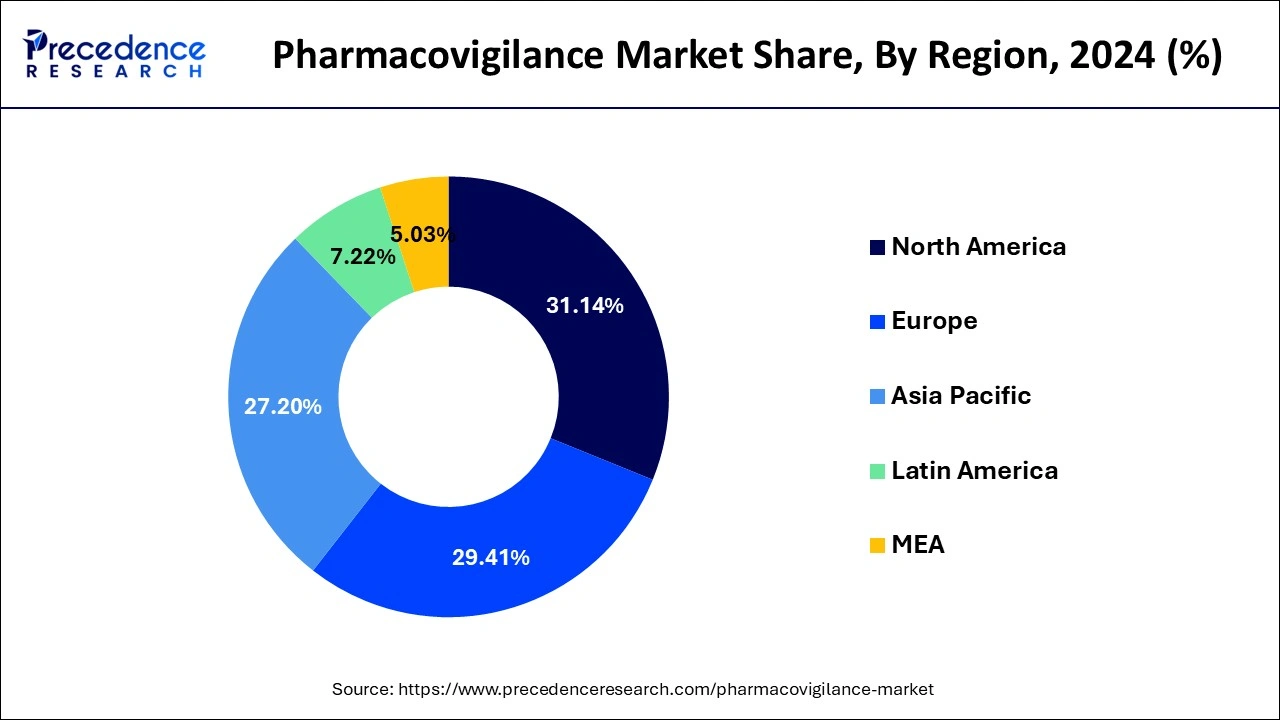
- North America market has generated 31.14% of the total revenue share in 2024.
- Asia Pacific region is expanding at a CAGR of 10.9% between 2025 and 2034.
- By end user, the pharmaceutical companies dominate the market with the largest revenue share 42.8% in 2024.
- By service provider, the contract outsourcing segment has generated a revenue share of 60% in 2024.
- By clinical trial phase, the phase IV segment has garnered a revenue share of 76% in 2024.
- By process flow, signal detection has held a revenue share of around 39.6% in 2024.
- By therapeutic area, the oncology segment has captured a revenue share of over 26.8% in 2024 with a CAGR of 11.2%.
- By type, spontaneous reporting has accounted revenue share of 30.4% in 2024.
Market Size and Forecast
- Market Size in 2025: USD 10.36 Billion
- Market Size in 2026: USD 11.29 Billion
- Forecasted Market Size by 2034: USD 22.25 Billion
- CAGR (2025-2034): 8.88%
- Largest Market in 2024: North America
- Fastest Growing Market: Asia Pacific
Role of Artificial Intelligence (AI) in pharmacovigilance
The rising applications of artificial intelligence (AI) in the pharmaceuticals and healthcare industries has led to adoption of AI into the pharmacovigilance market. AI technology helps in detection and assessment of potential adversities by detecting patterns through analyzation of vast amount of data from various sources. With AI technology, detection and assessment of adverse drug reactions helps professionals of the field make an informed decision. The integration of AI in pharmacovigilance services helps in boosting efficiency and effectiveness of the drug monitoring safety process. This market can expand even further with adoption of AI during the forecast period.
Market Overview
Pharmacovigilance has an important role to play in improvement of public health outcomes while helping regulatory decision to make sure the harm from medications is bare minimum. The rising cases of adverse drug reactions has created a necessity for proper drug safety monitoring. The demand for technologies and services that will track, manage and analyze drug safety data effectively are required. The different regulatory agencies across the world and rising adverse drug reactions is expected to boost the growth pharmacovigilance market in the forecast period.
Market Trends
- Growing Development and Consumption of Drugs: The growing expansion of the pharmaceutical industry in various regions is increasing the development of drugs is driving demand for pharmacovigilance systems. The growing chronic conditions increase the consumption of drugs, fueling demand for pharmacovigilance systems to monitor the adverse effects of drugs.
- Rising Awareness About Adverse Effects of Drugs: The growing awareness about the risk associated with medications among healthcare professionals and patients increases demand for a pharmacovigilance system. The strong focus on the safety of patients helps in the market growth.
- Technological Advancements: The ongoing integration of machine learning and artificial intelligence in pharmacovigilance systems helps in the market growth. Technological advancements enhance process automation, data analysis, & signal detection and lower manual workload.
Pharmacovigilance Market Growth Factors
- The rising prevalence of chronic diseases is expected to propel the market growth.
- The pharmacovigilance market is growing due to expanding usage of outsourcing services, increasing drug development rates, and rising drug consumption.
- The pharmaceutical industry's high spending, as well as an increase in adverse drug reactions (ADRs) and prescription errors, are propelling the market growth. The non-profit organization's growing awareness of pharmacovigilance may boost growth.
- The necessity for regular medication monitoring has increased as a result of rising drug consumption, supporting the pharmacovigilance market growth during the forecast period.
Pharmacovigilance Market Outlook
- Industry Growth Overview The pharmacovigilance market is expected to grow significantly between 2025 and 2034. This growth is driven by stricter regulation requirements, faster global clinical trial progress, and increased concern for patient safety across all regions. The rising complexity of pipelines for pharmaceuticals, biologics, gene therapies, and combination products is further boosting the need for specialized safety monitoring services. Regulatory demands, clinical trial complexity, and the adoption of new technology are all guiding the pharmacovigilance industry toward long-term, high-margin growth.
- Technology & Innovation Trends: The use of technology in pharmacovigilance is evolving, with AI, machine learning, natural language processing, and cloud computing enabling more effective case processing, predictive risk analysis, and signal detection. Advanced safety databases and pharmacovigilance systems allow adverse events to be monitored in real-time. This significantly reduces manual workload and enhances compliance with international regulatory standards. These innovations make the world's pharmacovigilance activities faster, smarter, and more scalable.
- Global Expansion:Leading pharmacovigilance service providers are actively expanding their global presence to meet increasing demand for drug safety services in emerging and high-growth markets. Several companies like IQVIA, Parexel, Syneos Health, and Covance are establishing new safety operations sites in India, China, Eastern Europe, and Latin America. They address regional clinical trial needs and regulatory compliance requirements. Additionally, the rising safety reporting demands on regulatory bodies make regional presence a key competitive advantage for major pharmacovigilance service providers.
- Major Investors:The pharmacovigilance sector is drawing considerable interest from private equity and strategic investors because of its recurring revenue models, regulatory significance, and strong growth opportunities. These investments are also supporting market consolidation, technological integration, and scaling of global service operations. Strategic investors are particularly interested in firms that combine regulatory expertise with advanced software platforms, positioning them as leaders in the next generation of safety monitoring.
- Startup Ecosystem:The pharmacovigilance startup ecosystem is rapidly growing and driven by innovations in AI-powered safety analytics, mobile-based patient reporting solutions, and real-world evidence generation. New companies like Clarigent Health (USA), DeepPharma (UK), and MedSafety (India) are developing scalable solutions. These reduce the number of cases handled manually, accelerate signal detection, and improve regulatory compliance. These startups not only introduce technological advances but also shape the future of pharmacovigilance operations worldwide, offering alternatives to traditional, labor-intensive processes.
MarketScope
| Report Coverage | Details |
| Market Size in 2025 | USD 10.36 Billion |
| Market Size in 2026 | USD 11.29 Billion |
| Market Size by 2034 | USD 22.25 Billion |
| Growth Rate from 2025 to 2034 | CAGR of 8.88% |
| Largest Market | North America |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | By Clinical Trial Phase, By Service Provider, and By End User, and Region |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers
Rising demand for contract outsourcing by pharmaceutical companies
The pharmacovigilance market is witnessing a major shiftthe pharmaceutical companies increasingly looking to outsourcing services for pharmacovigilance. These services offer the companies several benefits because it enables them to have access to a broader spectrum of resources, that include regulatory compliance, specialized management, safety reporting etc. The companies can also manage demand better with outsourcing while reducing costs and overhead expense. The preference towards outsourcing by key pharmaceuticals companies is driving market growth.
Restraint
Lack of skilled professionals and funding
The demand for the pharmacovigilance services is high. Although, there is a lack of funding along with shortage of professionals that are skilled and trained. This creates a major hurdle for the pharmacovigilance market. The insufficiency in funds lags the establishment of effective mechanisms and infrastructure to properly carry out pharmacovigilance services. There is a delay in reporting and correct monitoring because of lack of skilled professionals. This directly affects the effectiveness of this practice. The key players are trying figure out how to tackle these challenges in an effective way.
Opportunity
Expansion in emerging market
The emerging markets of Latin America and Asia Pacific are incredible opportunities of innovation and growth for the pharmacovigilance market. Both these regions are undergoing urbanization and economic development at a rapid pace. The huge population combined with growing spending capacity and government initiatives has helped propel the healthcare infrastructure. Many key players are looking to expand into these regions by setting research and development facilities close to a large pool of patients. The countries of these region offer this market an expansion and development opportunity.
Segment Insights
Clinical Trial Phase Insights
The phase IV segment has dominated the market with highest revenue share in 2024. The technologies used for clinical trial phase IV, provide as an extra layer of protection for medications in clinical studies. Phase IV is important and essential step in clinical trials as it allows for the detection of negative medication effects. As a result of thorough drug testing on large patient demographics of the highest relevance after commercialization of the drug, the data collected and reviewed during this stage is predicted to be of the highest relevance.
On the other hand, the phase III is expected to grow at rapid pace during the forecast period. Phase III trials are used to determine and confirm a drug's efficacy. Before a medicine is commercialized, these trials provide further information about probable drug interactions, effectiveness, and drug safety. Over the forecast period, the aforementioned factors are expected to drive revenue generation in the segment.
Service Provider Insights
The contract outsourcing segment has accounted highest revenue share of 60% in 2024. This is owing to the advantages of outsourcing, such as risk minimization, resource flexibility, lower fixed costs, and upfront investment reduction. The pharmacovigilance audits, standard operating procedures, and other specialized services are provided by contract outsourcing businesses.
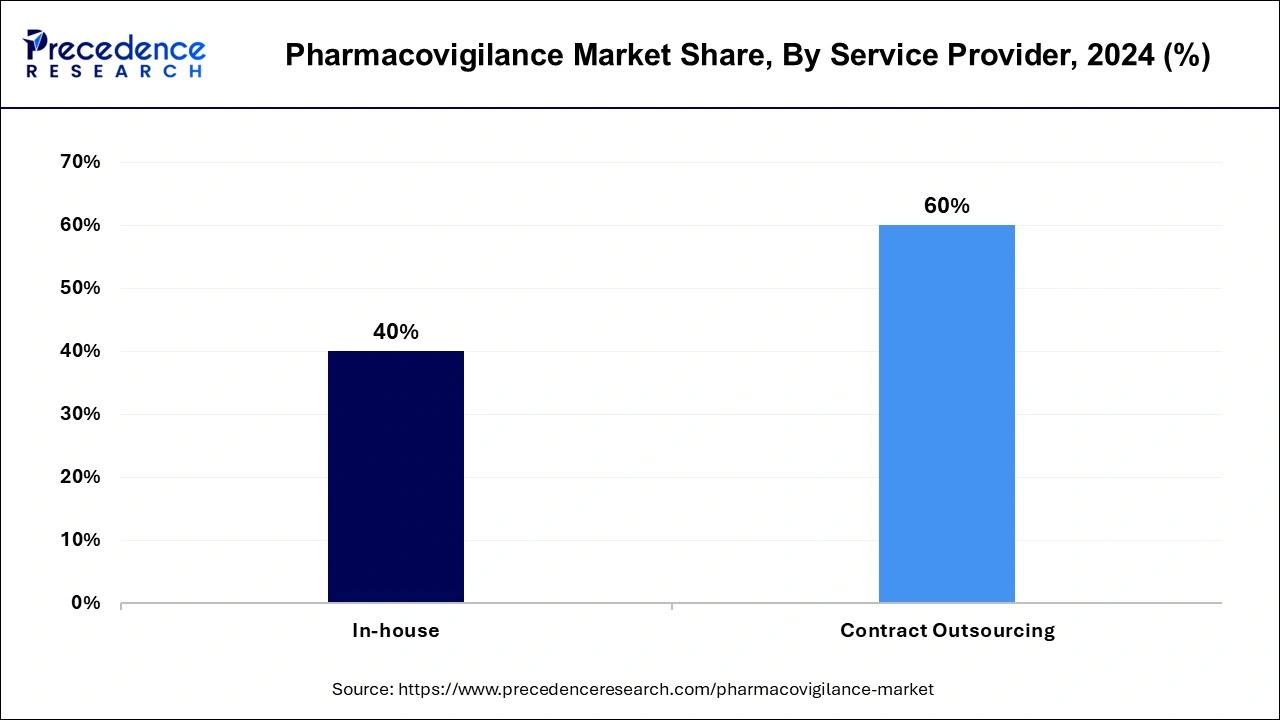
On the other hand, as a result of substantial research and development by large pharmaceutical and biotechnological businesses for the creation of new pharmaceuticals, the in-house segment is expected to rise moderately over the forecast period. In the following years, this is projected to benefit the industry's growth prospects.
End User Insights
Based on the end user, the pharmaceutical companies has hed highest revenue share in 2024. Due to increased new product development activities in this sector, the pharmaceutical companies segment is expected to rise significantly in the next years. Drug development and consumption have increased dramatically in recent years. The adverse effects not identified in clinical studies can occur when medications are used by a wide population for longer periods of time.
On the other hand, the hospitals segment is expected to grow at rapid pace during the forecast period. The hospitals and clinics have started to outsource the pharmacovigilance process to avoid large high initial investments and fixed overhead costs, gain additional capacity, and boost resource flexibility. The hospitals can save money by outsourcing pharmacovigilance.
Regional Insights
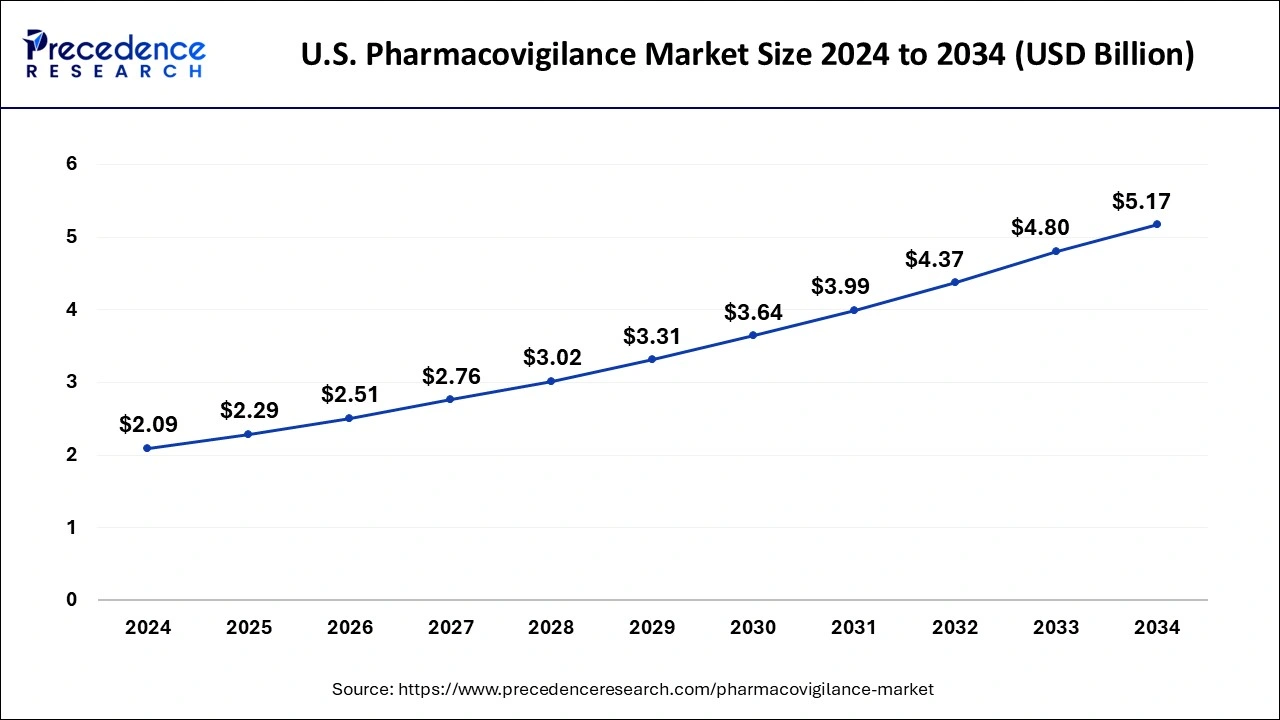
U.S. Pharmacovigilance Market Size and Growth 2025 to 2034
The U.S. pharmacovigilance market size is estimated at USD 2.29 billion in 2025 and is predicted to be worth around USD 5.17 billion by 2034, at a CAGR of 8.90% from 2025 to 2034.
Based on the region, the North America segment dominated the global pharmacovigilance market in 2024, in terms of revenue and is estimated to sustain its dominance during the forecast period. The increased prevalence of drug addiction and the resulting adverse drug reactions is a major cause of morbidity and mortality. This is a high-growing rendering factor for North American market growth. The regional market is expected to rise as key manufacturers increase their investments in novel drug development. As a result of the high volume of pharmaceuticals produced, the number of clinical studies and the demand for post-marketing supervision has increased, adding to the regional market's overall expansion.
U.S. is major contributor in the pharmacovigilance market in North America. Continuous drug safety monitoring is necessary to address the potential adverse effects in the large population of U.S. for rising chronic diseases like cancer, diabetes, heart disorders and hypertension. Stringent regulations set by the Food and Drug Administration (FDA) like the Risk Evaluation and Mitigation Strategies (REMS) program ensures strong pharmacovigilance practice are followed for improving drug safety and minimizing risks. Pharmaceutical companies are outsourcing pharmacovigilance activities for optimizing operational costs and maintain adherence to regulatory standards. Furthermore, increased emphasis on personalized medicine, growing use of biologics and use of advanced technologies such as automation and artificial intelligence (AI) are the factors driving the market growth.
- For instance, in June 2024, FDA's Center for Drug Evaluation and Research (CDER) established the Emerging Drug Safety Technology Program (EDSTP) which focuses on utilizing AI and other emerging technologies with a multifaceted approach in pharmacovigilance.
What Makes Asia Pacific the Fastest-Growing Region in the Market?
The Asia-Pacific is estimated to be the most opportunistic segment during the forecast period. In the coming years, greater productivity, resource sharing, and cost efficiency are expected to propel regional demand for pharmacovigilance. In addition, the regional market is being driven by expanding patient awareness, rising investment, and supportive government actions to address the needs of the population.
China Pharmacovigilance Market Trends
China is a major contributor to the pharmacovigilance market. The growing pharmaceutical industry and volume of drug development increase demand for pharmacovigilance. The growing incidence of adverse drug reactions helps in the market growth. The strong government support for drug safety increases focus on pharmacovigilance systems. The growing healthcare expenditure and presence of a large population increase the consumption of drugs, fueling demand for pharmacovigilance.
The increasing investment in advanced technologies like data analytics and electronic health records fuels the adoption of pharmacovigilance. Furthermore, the growing awareness of drug safety and the development of the biotechnology sector drive the overall market growth.
India's Pharmacovigilance Market Trends
India is significantly growing in the pharmacovigilance market. The growing expansion of the pharmaceutical industry increases demand for a pharmacovigilance system for monitoring drug safety. The rising regulatory oversight for strengthening drug safety through the Central Drugs Control Organization helps in the market growth.
The increasing awareness about risks associated with medications in healthcare professionals & patients increases demand for pharmacovigilance systems. The growing clinical trials and strong focus on patient safety support the overall growth of the market.
Why Europe is Growing in the Pharmacovigilance Market?
Europe is growing in the pharmacovigilance market. The stricter regulatory frameworks in the biotechnology and pharmaceutical industry increase demand for the pharmacovigilance market. The growing elderly population and rising chronic conditions & adverse drug reactions help in the market growth. The rising investment in the healthcare industry and services fuels demand for pharmacovigilance services. The growing demand for biosimilars, adaptive trial designs, personalized medicines, and orphan drugs increases demand for pharmacovigilance services. The growing technological advancements, like electronic health records, drive the overall growth of the market.
Germany Pharmacovigilance Market Trends
Germany saw the highest use of outsourced pharmacovigilance services in 2024. This was driven by pharmaceutical companies' tendency to outsource services to CROs for case processing, aggregate reporting, and risk management. Germany's interest in biologics, advanced therapeutics, and specialty drugs is also likely to increase demand for technology-driven PV services. It is expected that the strategic growth of global safety operations centers in Germany will help the country become a PV hub in Europe.
How is the Opportunistic Rise of Latin America in the Pharmacovigilance Market?
The Latin American pharmacovigilance market is expected to grow steadily in the coming years due to an increase in adverse event reporting and expanding regional CROs. Brazil and Mexico are the largest contributors because of intense clinical trials and regulatory modernization. The future of technology-driven pharmacovigilance efforts, such as AI-based case triaging, automated reporting, and cloud-based safety databases, is expected to lead the industry.
In 2024, case processing services were in high demand in Brazil due to the increasing reporting of adverse events and the regional growth of CRO activities. The regulatory modernization of ANVISA and the requirement for electronic submissions strengthened the need for efficient PV processes. It is expected that the expansion of multinational pharmaceutical operations in Brazil will boost the demand for an integrated PV platform in the region.
What Potentiates the Growth of the Middle East and Africa Pharmacovigilance Market?
The market in the Middle East & Africa is expected to grow due to the increasing establishment of regional drug safety monitoring centers. Other countries leading in the adoption of standardized reporting systems include UAE and South Africa, which have improved their compliance with international safety standards. The implementation of real-time pharmacovigilance is likely to be supported by investments in digital health infrastructure, such as electronic medical records and mobile patient reporting.
In 2024, adverse event reporting was the dominant factor in the UAE pharmacovigilance market due to the increasing number of imported pharmaceutical products and the establishment of regional safety monitoring centers. International safety regulations and data quality standards were enhanced with the adoption of standardized reporting systems. The enforcement of post-marketing surveillance and patient safety regulations is expected to boost the adoption of technology-driven pharmacovigilance solutions.
Value Chain Analysis
- R&D: This stage deals with the discovery and development of new drugs or the possible enrichment of the existing drug formulation; hence, it is the fountainhead for pharmaceutical innovations.
Key Players: Pfizer, Roche - Clinical Trials and Regulatory Approvals: These are tests on human beings of potentially new drugs, after the confirmation of their safety and effectiveness, and governmental approvals have to be secured before their release on the market.
Key Players: IQVIA, ICON plc - Formulation and Final Dosage Preparation: These are the steps of preparing chemical and biological compounds to manufacture drugs while taking care of quality and scale issues.
Key Players: Sun Pharmaceutical Industries, Cipla - Packaging and Serialization: Packaging involves designing labels and protective casing for medicines, while serialization involves the assignment of unique identification numbers to each unit to enable tracking and to prevent counterfeiting.
Key Players: MM Packaging, Aphena Pharma Solutions - Distributing to Hospitals and Pharmacies: This concerns the processes to ensure the delivery of the right pharmaceutical products to health care providers right on time so that they are available and accessible to patients.
Key Players: McKesson Corporation, Cardinal Health - Patient Support and Pharmacovigilance Services: Activities intended to improve patient outcomes and safety, including the monitoring of adverse drug reactions and ensuring the efficacy of preventive remedies.
Key Players: IQVIA, ICON plc
Pharmacovigilance Market Companies
- ICON Plc (Ireland) – A global leader in clinical research and pharmacovigilance services, ICON integrates advanced analytics and automation to enhance drug safety monitoring across global trials.
- Pharmaceutical Product Development LLC (USA)– PPD delivers end-to-end pharmacovigilance and risk management services, leveraging real-world evidence to improve post-market safety evaluations.
- Parexel International Corporation (USA) – Parexel is recognized for its AI-powered safety data management and global regulatory compliance expertise in pharmacovigilance.
- IQVIA (USA)– IQVIA dominates the pharmacovigilance space with real-time safety signal detection powered by AI, big data, and its vast healthcare intelligence network.
- Quanticate (UK) – Quanticate provides specialized pharmacovigilance and biostatistical services, focusing on high-quality adverse event data processing and regulatory submissions.
- Bioclinica (USA) – Bioclinica offers integrated drug safety, clinical data, and regulatory solutions that streamline signal detection and global case management.
- Covance Inc. (USA) – Covance, part of Labcorp, supports comprehensive pharmacovigilance operations from preclinical safety to post-market surveillance with global regulatory reach.
- Accenture Plc (Ireland)– Accenture transforms pharmacovigilance through digital automation, intelligent case processing, and predictive safety analytics.
- IBM Corporation (USA)– IBM brings cognitive computing to pharmacovigilance, applying Watson AI for automated signal detection and safety data interpretation.
- Novartis (Switzerland) – Novartis combines in-house pharmacovigilance expertise with AI and real-world data integration to enhance global drug safety surveillance.
Recent Developments
- On July 23, 2025, the Pistoia Alliance launched two groundbreaking initiatives to revolutionize pharmacovigilance through AI-driven innovation. Its new AI in Safety & PV Community of Expertsguide the ethical and effective application of AI, while the Pharmacovigilance Systems & Processes Standards (PS²) project aims to unify global PV system standards. Supported by Novo Nordisk, AbbVie, and Novartis, these projects promote collaboration, standardization, and technological advancement in drug safety.
- On July 17, 2025, EVERSANA introduced EVERSANA ORCHESTRATE PV, an AI-powered solution redefining pharmacovigilance efficiency. The platform streamlines key workflows like literature monitoring and aggregate reporting, achieving up to 50% faster operations with 40% less manual effort. This unified solution exemplifies EVERSANA's commitment to digital transformation and operational excellence in drug safety management.
Segments Covered in the Report
By Clinical Trial Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Service Provider
- In-house
- Contract Outsourcing
By End User
- Hospitals
- Pharmaceutical Companies
- Others
By Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
By Type
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
By Process Flow
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing & Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review & Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
By Region
- North America
- Europe
- Asia Pacific
- Middle East & Africa
- Latin America
For inquiries regarding discounts, bulk purchases, or customization requests, please contact us at sales@precedenceresearch.com
Frequently Asked Questions
Ask For Sample
No cookie-cutter, only authentic analysis – take the 1st step to become a Precedence Research client



